262
Orgnofluorine chemistry: Difluoromethylene motifs spaced 1,3 to each other imparts facial polarity to cyclohexane ring.
Mathew J. Jones, Ricardo Callejo, Alexandra M. Z. Slawin, Michael Bühl and David O’Hagan
Beilstein J. Org. Chem., 2016, 12, 2823–2827
10.3762/bjoc.12.281
261
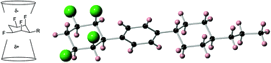 Fluorinated liquid crystals: Evaluation of selectively fluorinated facially polarised cyclohexyl motifs for liquid crystal applications.
Fluorinated liquid crystals: Evaluation of selectively fluorinated facially polarised cyclohexyl motifs for liquid crystal applications.
Nawaf Al-Maharik, Peer Kirsch, Alexandra M. Z. Slawin, David B. Cordes and David O’Hagan
Org. Biol. Chem., 2016, 14, 9974–9980
10.1039/C6OB01986B
260
Last-step enzymatic [18F]-fluorination of cysteine-tethered RGD peptides using modified Barbas linkers.
Qingzhi Zhang, Sergio Dall’Angelo, Ian N. Fleming, Lutz F. Schweiger, Matteo Zanda, David O’Hagan
Chem. Eur. J., 2016, 22, 10998–11004
10.1002/chem.201601361
 259
259
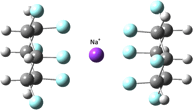
Janus Face Aspect of All-cis 1,2,3,4,5,6-Hexafluorocyclohexane Dictates Remarkable Anion and Cation Interactions In the Gas Phase.
Blake E. Ziegler, Michael Lecours, Rick A. Marta, Joshua Featherstone, Eric Fillion, W. Scott Hopkins, Vincent Steinmetz, Neil S. Keddie, David O’Hagan and Terrance B. McMahon
J. Am. Chem. Soc., 2016, 138, 7460–7463
10.1021/jacs.6b02856
Highlighted in:
ACS C&EN News
258
Fluorinated Musk Fragrances: The CF2 Group as a Conformational Bias Influencing the Odour of Civetone and (R)-Muscone.
Ricardo Callejo, Michael J. Corr, Mingyan Yang, Mingan A. Wang, David B. Cordes, Alexandra M. Z. Slawin and David O’Hagan
Chem. Eur. J., 2016, 22, 8137–8151
10.1002/chem.201600519
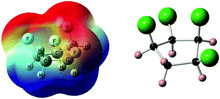 257
257
Polar alicyclic rings: synthesis and structure of all cis-1,2,3,4-tetrafluorocyclopentane.
Zeguo Fang, Nawaf Al-Maharik, Alexandra M. Z. Slawin, Michael Bühl and David O’Hagan
Chem. Commun., 2016, 52, 5116–6119.
10.1039/C6CC01348A
 256
256
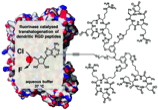
Enzymatic transhalogenation of dendritic RGD peptide constructs with the fluorinase.
Stephen Thompson, Ian N. Fleming and David O’Hagan
Org. Biomol. Chem., 2016, 14, 3120–3129
10.1039/C6OB00239K
 255
255
Accurate Lipophilicity (log P) Measurements Inform on Subtle Stereoelectronic Effects in Fluorine Chemistry.
David O’Hagan and Robert J. Young
Angew. Chem. Int. Ed., 2016, 55, 3858–3860
10.1002/anie.201511055
254
Common Phenolic Metabolites of Flavonoids, but Not Their Unmetabolized Precursors, Reduce the Secretion of Vascular Cellular Adhesion Molecules by Human Endothelial Cells.
Emily F. Warner, Qingzhi Zhang, K. Saki Rahwm, David O’Hagan, Maria A. O’Connell and Colin D. Kay
J. Nutr., 2016, 146, 3, 465–473
10.3945/jn.115.217943
 253
253
Exploration of a potential difluoromethyl-nucleoside substrate with the fluorinase enzyme.
Stephen Thompson, Stephen A. McMahon, James H. Naismith and David O’Hagan
Bioorg. Chem., 2016, 64, 37–41
10.1016/j.bioorg.2015.11.003
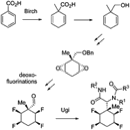 252
252
Multicomponent reactions of methyl substituted all-cis tetrafluorocyclohexane aldehydes.
Tetiana Bykova, Nawaf Al-Maharik, Alexandra M. Z. Slawin and David O’Hagan
Org. Biomol. Chem., 2016,14, 1117–1123
10.1039/C5OB02334C
251
Inter- and intramolecular CF···C=O interactions on aliphatic and cyclohexane carbonyl derivatives.
Rodrigo A. Cormanich, Roberto Rittner, David O’Hagan and Michael Bühl
J. Comp. Chem., 2016, 37, SI 25–33
10.1002/jcc.23918
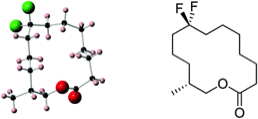 250
250
Fluorine in fragrances: exploring the difluoromethylene (CF2) group as a conformational constraint in macrocyclic musk lactones.
Michael J. Corr, Rodrigo A. Cormanich, Cortney N. von Hahmann, Michael Bühl, David B. Cordes, Alexandra M. Z. Slawin and David O’Hagan
Org. Biomol. Chem., 2016, 14, 211–219
10.1039/C5OB02023A
